SV Spectrum™ catheters are
*Currently branded and sold as Cook Spectrum® Technology
The Right Combination
Dr. Darouiche and Dr. Raad discovered that the pairing of minocycline and rifampin (M+R) produced a combination that is effective at protecting against a broad spectrum of organisms, including CNS, MRSA, VRE, VRSA, and several gram-negative bacteria.
Minocycline+rifampin is the most rigorously studied combination of antibiotics available on a catheter for reducing CLABSIs/CRBSIs through two distinct mechanisms of action. “Coated catheters with zone sizes of ≥ 15 mm were highly predictive of in vivo efficacy, whereby colonization of the indwelling catheter is prevented.”1,2
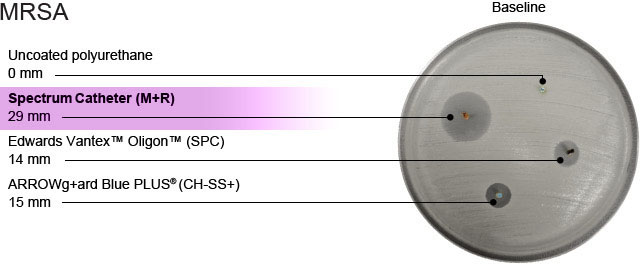
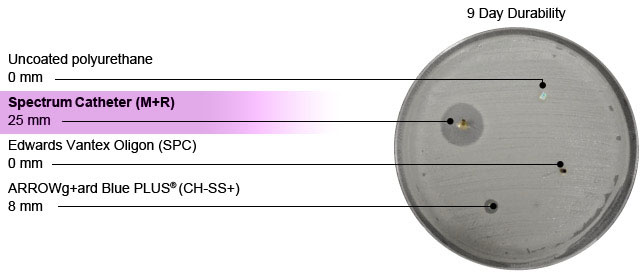
Source: I. Raad, MD, Chair, Department of Infectious Diseases. M.D. Anderson Cancer Center, University of Texas School of Medicine, Houston, Texas.
REFERENCES
- Raad I, Darouiche R, Hachem R, Sacilowski M, Bodey GP. Antibiotics and prevention of microbial colonization of catheters. Antimicrob Agents Chemother. 1995;39(11):2397-2400. doi:10.1128/AAC.39.11.2397
- Sherertz RJ, Carruth WA, Hampton AA, Byron MP, Solomon DD. Efficacy of antibiotic-coated catheters in preventing subcutaneous Staphylococcus aureus infection in rabbits. J Infect Dis. 1993;167(1):98-106. doi:10.1093/infdis/167.1.98
The Evidence

Two decades of evidence, including over 30 peer-reviewed studies and meta-analyses, confirm that minocycline and rifampin (M+R) catheters are an effective tool to provide broad-spectrum protections against gram-positive and gram-negative infections for both short- and long-term use.
Process Plus Antimicrobial Catheter Technology

REFERENCE
- Bonne S, Mazuski JE, Sona C, et al. Effectiveness of Minocycline and Rifampin vs Chlorhexidine and Silver Sulfadiazine-Impregnated Central Venous Catheters in Preventing Central Line-Associated Bloodstream Infection in a High-Volume Academic Intensive Care Unit: A Before and after Trial.J Am Coll Surg. 2015;221(3):739-747. doi:10.1016/j.jamcollsurg.2015.05.013
*Currently branded and sold as Cook Spectrum® Technology.
No Evidence of Increased Resistance1

Five prospective studies have examined the susceptibility of clinical isolates to the development of antibiotic resistance from M+R CVC use. None of the studies showed any association between M+R and antibiotic resistance, nor did a 7-year study of over 500,000 catheter days.1


REFERENCES
- Ramos ER, Reitzel R, Jiang Y, et al. Clinical effectiveness and risk of emerging resistance associated with prolonged use of antibiotic-impregnated catheters: more than 0.5 million catheter days and 7 years of clinical experience.Crit Care Med. 2011;39(2):245-251. doi:10.1097/CCM.0b013e3181feb83e
Savings Calculator
Infection rates don’t have to be high for SV Spectrum™ Technology* to be a smart choice for your facility. CLABSIs/CRBSIs are among the most expensive healthcare-associated infections (HAIs) per case4 so even incremental reductions in CLABSIs/CRBSIs can create significant savings. By switching to SV Spectrum™ Technology*, even high-performing hospitals with low infection rates can achieve substantial reductions in mortalities and cost that are associated with infections.
Plus, by reducing your CRBSI rate, SV Spectrum™ Technology* can help your hospital lower the risk for a Hospital-Acquired Condition (HAC) Reduction Program score that triggers a 1% reduction in inpatient Medicare reimbursement.
Consider a facility that places 100 CVCs per month, using these numbers from the CDC3 and from recent studies1,2 that detail the toll of CRBSIs:
Acute CVCs
Annual catheterizations and infections
| CVCs Placed Annually 1200 | Total CRBSIs Annually 2828 |
|
| Average Dwell Time1 7 days | Average Excess Length of Stay5 8 days | Average Cost to Treat CRBSI3 $45,814.00 |
| Annual Catheter Days 8400 | Bed Days Spent to Treat CRBSIs 224 | Cost to Treat CRBSIs Annually 1,282,792.00 |
Based on your entries on the previous table, the impact of a 50% reduction in the CRBSI rate is shown below.
| Infection Rate Per 1000 Catheter Days 1.65 | Total CRBSIs 14 |
| Avg. Time Saved per Catheter 2h 14m | Avg. Dollars Saved per Catheter $534.50 |
| Bed Days Saved Annually 112 | Dollars Saved Annually $ 641,396.00 |
Average Dwell Time = 7 Days1
Average Excess Length of Stay = 8 Days5
Average Cost to Treat CRBSI = $458143
With Spectrum plus your process bundle, we can help you reach for that goal.
REFERENCES
- Ramos ER, Reitzel R, Jiang Y, et al. Clinical effectiveness and risk of emerging resistance associated with prolonged use of antibiotic-impregnated catheters: more than 0.5 million catheter days and 7 years of clinical experience.Crit Care Med. 2011;39(2):245-251. doi:10.1097/CCM.0b013e3181feb83e
- Advani S, Reich NG, Sengupta A, Gosey L, Milstone AM. Central line-associated bloodstream infection in hospitalized children with peripherally inserted central venous catheters: extending risk analyses outside the intensive care unit.Clin Infect Dis. 2011;52(9):1108-1115. doi:10.1093/cid/cir145
- Zimlichman, E, Henderson D, Tamir O, et al. Health care-associated infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. The $45,814 figure stated in the above calculator is the average between $30,919-$65,245, as referenced in this article.
- Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039-2046. doi:10.1001/jamainternmed.2013.9763
- Centers for Disease Control and Prevention (CDC). Vital signs: central line-associated blood stream infections–United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):243-248.
Frequently Asked Questions
What is SV Spectrum™ Technology* effective against?
Is SV Spectrum™ Technology* effective against fungus?
There is published in-vitro evidence demonstrating antimicrobial activity against specific strains of fungal organisms. However, to date, no published in-vivo evidence exists in humans demonstrating a true impact on the incidence of fungal infections from SV Spectrum™ Technology* or any current antimicrobial intravascular catheter.
Where can I find the studies that support the use of SV Spectrum™ Technology*?
Links to the studies’ abstracts can be found under References or Guidelines or by contacting your local Spectrum Vascular representative.
Do SV Spectrum™ Catheters* have any contraindications?
Allergy or history of allergy to tetracyclines or rifampin NOTE: Because SV Spectrum™ Catheters* are impregnated with a combination of the antimicrobial agents minocycline (a derivative of tetracycline) and rifampin (a derivative of rifamycin B), the contraindications, warnings, and precautions regarding use of these antimicrobials apply and should be adhered to for use of this device, although systemic levels of minocycline and rifampin in patients receiving this device are highly unlikely to result from their use. Minocycline and rifampin are agents that do not induce any genotoxic risk except a possible teratogenic effect in pregnant women. We therefore do not recommend the use of SV Spectrum™ Catheters* in pregnant women.
For how long is SV Spectrum™ Technology* effective against microbes that cause CRBSIS?
The duration of SV Spectrum™ Technology*’s effectiveness depends on the bacterium. Against methicillin-resistant Staphylococcus aureus (MRSA), it has antimicrobial durability of at least 35 days.1 Against vancomycin resistant Staphylococcus aureus (VRSA), MDR A. baumannii/calcoaceticus, MDR E. agglomerans, and MDR S. maltophilia, it has antimicrobial durability of at least 28 days.2 Against coagulase-negative Staphylococci (CNS), the half-life of the inhibitory activity of catheters coated with minocycline and rifampin was 25 days.3
Are there any antibiotic-resistance issues?
Over 20 years of clinical use have shown no evidence that minocycline and rifampin (M+R) catheters lead to increased bacterial resistance, and a 7 year study of over 500,000 catheter days confirms these results. 4
Do I need to use a special technique in placement or care?
Care for SV Spectrum™ Catheters* just as you would any catheter. It is recommended that you follow maximum sterile barrier precautions during placement and follow your institution’s protocol to care for the catheter.
Do I still need to use my standard patient prep process and postcare practices?
Yes, follow all of the normal practices that your institution has in place, including, but not limited to, proper skin prep, dressing changes, and best practices for accessing and flushing.
Are SV Spectrum™ Catheters* compatible with all antibiotics and other therapies?
SV Spectrum™ Catheters* are compatible with all standard infusates.
Can antiseptic dressings be used with SV Spectrum™ Catheters*?
Yes. Antiseptic dressings are compatible with minocycline and rifampin – the antibiotics impregnated within the catheters. The additional benefit of using antiseptic dressings with SV Spectrum™ Catheters* has not been studied.
Will SV Spectrum™ Catheters* treat a current infection?
No, SV Spectrum™ Catheters* are only for the prevention of CRBSIs, not for the treatment of an active infection.
If the patient gets a CRBSI while being treated with an SV Spectrum™ Catheter*, does it prohibit standard care?
No, neither minocycline nor rifampin are current mainstream antibiotics in the treatment of bloodstream infections, so they will not inhibit the use of your institution’s normal antibiotic therapies.
Is Minocycline or Rifampin detectable in the bloodstream?
No. Since the antibiotics are impregnated into the material, they are released slowly over time and are not detectable in the patient’s blood. The maximum amount of antibiotic present in a catheter is 1/10 of one systemic dose for normal therapies.5
Which types of catheters are available with SV Spectrum™ Technology*?
Spectrum Vascular provides different types of central venous catheters with SV Spectrum™ Technology*. These include PICCs and acute CVCs. Please contact your local Spectrum Vascular representative for further details.
Where was SV Spectrum™ Technology* developed?
The process of impregnating catheters with minocycline and rifampin was developed by a team led by Dr. Issam Raad and Dr. Rabih Darouiche. Dr. Raad is chair of infectious disease at M.D. Anderson Cancer Center in Houston, Texas.
Dr. Darouiche is chair of infectious disease at the Medical College of Baylor in Houston, Texas.
Can SV Spectrum™ Catheters* be used for power-injection?
Yes, several configurations can be used for power injection.
Does Spectrum Vascular provide education on catheters to healthcare professionals?
Yes. Spectrum Vascular provides training and support through in-servicing and custom workshops. We work with experts in the field to offer educational opportunities that will help you and your team keep up on the latest trends in medicine. Training is offered on a variety of topics and can be tailored to a format that fits your needs.
Where can I find guidelines for infection prevention and patient safety?
You can find a list of guidelines for infection prevention and patient safety here.
References
- Hanna H, Benjamin R, Chatzinikolaou I, et al. Long-term silicone central venous catheters impregnated with minocycline and rifampin decrease rates of catheter-related bloodstream infection in cancer patients: a prospective randomized clinical trial. J Clin Oncol. 2004;22(15):3163-3171.
- Raad I, Reitzel R, Jiang Y, et al. Anti-adherence activity and antimicrobial durability of anti-infective-coated catheters against multidrug-resistant bacteria. J Antimicrob Chemother. 2008;62(4):746-750.
- Raad I, Darouiche R, Hachem R, et al. The broad-spectrum activity and efficacy of catheters coated with minocycline and rifampin. J Infect Dis. 1996;173(2):418-424.
- Ramos ER, Reitzel R, Jiang Y, et al. Clinical effectiveness and risk of emerging resistance associated with prolonged use of antibiotic-impregnated catheters: more than 0.5 million catheter days and 7 years of clinical experience. Crit Care Med. 2011;39(2): 245-251.
- Raad I, Darouiche R, Dupuis J, et al. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections: a randomized, double-blind trial. Ann Intern Med. 1997;127(4):267-274.
*Currently branded and sold as Cook Spectrum® Technology.
International Guidelines for Infection Prevention and Patient Safety
Reputable published guidelines and standards for catheter care and management are available from several societies and health organizations. Links to these organizations are provided here for your reference.
| Name of Guideline | Organization | Website |
|---|---|---|
| Access Device Standards of Practice for Oncology Nursing (2017). [U.S.] | Oncology Nursing Society (ONS) | www.ons.org |
| Infusion Therapy Standards of Practice (2016). [U.S.] | Infusion Nurses Society (INS) | www.INS1.org |
| Advancing Excellence in Health Care: Recommendations for Patient Safety and Infection Prevention (2012). [U.S.] | Agency for Healthcare Research and Quality (AHRQ) | www.ahrq.gov |
| How-to Guide: Prevent Central Line-Associated Bloodstream Infection (2012). [U.S.] | Institute for Healthcare Improvement (IHI) | www.IHI.org |
| Guidelines for the Prevention of Intravascular Catheter-Related Infections (2011). [U.S.] | Interagency report | www.cdc.gov |
| Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infection (2009). [U.S.] | Infectious Diseases Society of America (IDSA) | www.idsociety.org |
| National Healthcare Safety Network (NHSN) Manual: Patient Safety Component Protocol (2009). [U.S.] | Centers for Disease Control (CDC) | www.cdc.gov |
| Nursing Best Practice Guideline: Care and Maintenance to Reduce Vascular Access Complications (2005). [CAN] | Registered Nurses' Association of Ontario (RNAO) | www.rnao.org |
| Standards for Infusion Therapy (2016). [U.K.] | Royal College of Nursing (RCN) | www.rcn.org.uk |
| epic3: National Evidence-Based Guidelines for Preventing Healthcare-Associated Infections in NHS Hospitals (2014). [U.K.] | Journal of Hospital Infection, commissioned by Department of Health (UK) | www.journalofhospitalinfection.com |
| Infection Control: Prevention of Healthcare–Associated Infections in Primary and Community Care (2012). [U.K.] | National Institute for Health and Clinical Excellence (NICE) | www.nice.org.uk |
| ESPEN Guidelines on Parenteral Nutrition: Central Venous Catheters (Access, Care, Diagnosis and Therapy of Complications) (2009). [EU] | European Society of Clinical Nutrition and Metabolism (ESPEN) | www.clinicalnutritionjournal.com |
*Currently branded and sold as Cook Spectrum® Technology.